
Roles of Informatics Pharmacists in Digital Health Interoperability
#SavoniaUAS
The International Pharmaceutical Federation (FIP) is a global organ that represents pharmacy, pharmaceutical sciences, and pharmaceutical education. FIP was established in 1912 and it has 146 organisational members around the world. In addition, there are several academic institutional members and individual members. FIP collaborates for example with World Health Organisation and UNESCO. (International Pharmaceutical Federation, 2021a.)
The vision of FIP is that everyone in the world has access to safe, effective, quality, and inexpensive medicines. Also, health technologies should be available for everyone. Furthermore, the pharmaceutical care services have provided by pharmacists, in cooperation with other healthcare professionals. FIP’s mission is to enhance global health with the pharmaceutical practice, sciences, and education. (International Pharmaceutical Federation, 2021a.)
In September 2021 FIP released a statement of policy on the importance of interoperability in the pursuit of a successful global digital health implementation (International Pharmaceutical Federation, 2021b). The policy brings attention to two key roles informatics pharmacists can play in designing and reinforcing best practices in medication-related interoperability.
The first role is to assist in creating and establishing semantic interoperability related to medication data. Semantic interoperability pertains to concepts and standards developed for usage across clinical digital systems, providing a standard digital language vocabulary for health terminology that can be comprehended by both people and computers (Lehne et al., 2019). Medication standards need to be designed according to the country’s pharmaceutical product regulatory practices and policies. For example, the National Library of Medicine (NLM) produced RxNorm, a standardized naming system for generic and branded medications registered in the United States of America (National Library of Medicine, n.d.). NLM also provides relational diagrams to link relevant drug concepts, such as the active ingredient of drugs, dosage form, and brand name, enabling robust navigation and adaptation of the unique identifiers found within the system (National Library of Medicine, n.d.). Figure 1 illustrates a RxNorm relational chart for Cetrizine (a medication used in allergic rhinitis).
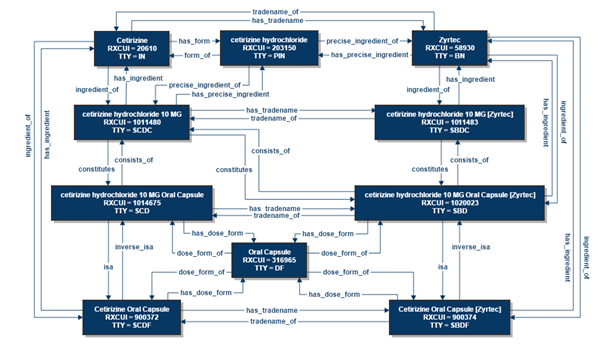
As a part of licensing requirements to practice as pharmacists, informatics pharmacists need to be familiar with local pharmaceutical product regulatory practices and policies. Informatics pharmacists also possess working-level knowledge in information technology, usually obtained through post-graduate studies and on-the-job training. They are well-positioned to participate in medication standards projects as essential team members.
The second role is to curate guidelines, policies, and regulations that support organizational interoperability. Organizational interoperability refers to high-level efforts to encourage and enforce interoperability at the institutional, national, and global levels. Since informatics pharmacists are familiar with medication standards and best practices in ensuring safety in medication use processes, their expertise is crucial in implementing organizational interoperability. For example, they should be involved in writing editorial guidelines dictating the configuration of medication records in electronic health records.
The two roles described above illustrate potential opportunities available for informatics pharmacists. As the global healthcare industries are moving toward digitalization, it is time for pharmacists to evaluate their skill sets and consider positions where they can have the most significant impact on patient and general public health outcomes.
Franky Franky, Master’s Degree Programme in Digital Health Student, Savonia University of Applied Sciences
Liisa Klemola, Lecturer, PhD
Savonia University of Applied Sciences, Unit of Continuous Learning, Master School, Kuopio
Picture source: Pixabay
References:
International Pharmaceutical Federation. (2021a). International Pharmaceutical Federation. https://www.fip.org/
International Pharmaceutical Federation. (2021b). FIP Statement of Policy on Digital health. https://www.fip.org/file/5092
Lehne, M., Sass, J., Essenwanger, A., Schepers, J., & Thun, S. (2019). Why digital medicine depends on interoperability. Npj Digital Medicine, 2(1). https://doi.org/10.1038/s41746-019-0158-1
National Library of Medicine. (n.d.). RxNorm Overview. Retrieved April 30, 2022, from https://www.nlm.nih.gov/research/umls/rxnorm/overview.html